#pharmacovigilance companies
Explore tagged Tumblr posts
Text
Why is Data Critical in Life Sciences and the Emerging Trends in 2024
The life sciences industry covers sectors such as pharmaceuticals, biotechnology, healthcare, and medical devices, and the sector has been rapidly evolving in the last few years based on the interaction of data analytics, AI, and other corresponding digital technologies. The growth of precision medicine, a desire for better efficiency in drug development, and a growing focus on patient outcomes are part of the reason why data has become so critical to innovation in the life sciences industry today.
#API manufacturers in India#pharmaceutical software companies#pharmacovigilance companies#pharma software companies#pharmacovigilance services#pharmaceutical companies in Singapore#data science in pharmaceutical industry#pharmaceutical companies in UAE
1 note
·
View note
Text
Pharmacovigilance Services in USA | MedVoice
Pharmacovigilance Services in USA | MedVoice Empower your drug discovery process, enhance research accuracy, and elevate product quality with MedVoice's meticulous pharmacovigilance services. Pharmacovigilance Services, Pharmacovigilance Solutions, Pharmacovigilance Services Providing Company, Pharmacovigilance In USA
#Pharmacovigilance Services#Pharmacovigilance Solutions#Pharmacovigilance Services Providing Company#Pharmacovigilance In USA
1 note
·
View note
Link

Lambda is one of the best Drug Safety and Pharmacovigilance services to our regional customers as per client requirements. Our teams are focused on safety in clinical trials and other activities and are ready to help with your project.
0 notes
Text
What is Pharmacovigilance? Understanding Drug Safety and Risk Management

What is Pharmacovigilance? Pharmacovigilance (PV) is the science and practice of detecting, assessing, understanding, and preventing adverse effects or other drug-related problems. Its primary goal is to ensure that the benefits of medicinal products outweigh their risks, thus safeguarding public health.
This discipline extends across the entire lifecycle of a drug, from pre-market clinical trials to post-marketing surveillance, encompassing the global monitoring of medicinal products.
Core Objectives of Pharmacovigilance Identifying Adverse Drug Reactions (ADRs): PV seeks to detect adverse effects, especially those that are unexpected or serious, arising from the use of medicinal products.
Ensuring Drug Safety: By monitoring and managing risks, pharmacovigilance contributes to the safer use of medicines.
Educating Stakeholders: Healthcare providers, regulatory authorities, and patients are informed about potential risks and proper usage of drugs.
Regulatory Compliance: Pharmaceutical companies must adhere to international standards and report safety data to regulatory bodies like the FDA, EMA, and WHO.
Importance of Pharmacovigilance Protecting Patient Health: By identifying potential risks early, pharmacovigilance minimizes harm to patients and ensures the safety of medicinal products.
Supporting Regulatory Decisions: Comprehensive safety data help regulatory agencies make informed decisions about the approval, withdrawal, or restriction of drugs.
Enhancing Pharmaceutical Innovation: A robust PV framework fosters confidence in the pharmaceutical industry, encouraging innovation while ensuring public safety.
Global Harmonization: Pharmacovigilance facilitates uniform safety standards across countries, promoting international cooperation in drug safety monitoring.
Challenges in Pharmacovigilance Data Overload: The influx of safety data from clinical trials, real-world use, and social media presents challenges in effective monitoring.
Complex Regulations: Varying regulatory requirements across countries require significant expertise to navigate.
Technological Integration: Adopting advanced tools like AI and machine learning for signal detection and data analysis is a complex but necessary step forward.
Public Awareness: Educating patients and healthcare professionals about reporting adverse events remains a significant hurdle.
Technological Advances in Pharmacovigilance The integration of technology is revolutionizing pharmacovigilance. Tools such as artificial intelligence (AI), big data analytics, and machine learning are enhancing signal detection, enabling quicker identification of potential risks. Real-world evidence from wearable devices and electronic health records (EHRs) provides real-time insights into drug safety.
The Future of Pharmacovigilance As global healthcare systems evolve, pharmacovigilance will play an increasingly pivotal role. The focus will shift towards personalized medicine, where monitoring individual patient responses to drugs will become central. Additionally, greater emphasis on patient engagement and transparent communication will redefine the relationship between healthcare providers and patients.
Conclusion Pharmacovigilance is the cornerstone of drug safety, ensuring that the medicines we rely on are both effective and safe. By bridging the gap between pharmaceutical innovation and patient safety, it upholds the integrity of healthcare systems worldwide.
As technology and global collaboration continue to advance, pharmacovigilance will remain a critical field in protecting and improving public health, shaping a safer and more informed future for all.
#pharmacovigilance service#pharmacovigilance#clinic#clinical research#clinical trial management#clinical trial operations#clinical trial monitoring
2 notes
·
View notes
Text
Paul Hennessy/SOPA/Getty Images
June 14, 2023
Horowitz: Confidential Pfizer document shows the company observed 1.6 million adverse events covering nearly every organ system
Daniel Horowitz
Over 10,000 categories of nearly 1.6 million adverse events – many of them serious and debilitating – brought to you by Pfizer!
You might not have heard it in the news, but in recent months, Pfizer’s pharmacovigilance documents requested by the European Union’s drug regulator, the European Medicines Agency, have been released. They show that Pfizer knew about a sickening level of injury early on. An August 2022 document shows that the company already had observed the following scope of vaccine injury:
508,351 individual case reports of adverse events containing 1,597,673 events;
One-third of the AEs were classified as serious, well above the standard for safety signals usually pegged at 15%;
Women reported AEs at three times the rate of men;
60% of cases were reported with either “outcome unknown” or “not recovered,” so many of the injuries were not transient;
Highest number of cases occurred in the 31-50 year age group, and 92% did not have any comorbidities, which makes it very likely it was the vaccine causing such widespread, sudden injury.
These numbers alone suggest that all COVID shots should be defunded and Congress must immediately remove liability protections from the manufacturers. But a more recent document released by the Europeans is even more devastating, because it breaks down the 1.6 million adverse events observed by Pfizer by category and subcategory of ailment and injury.
The 393-page confidential Pfizer document, dated Aug. 19, 2022, shows that Pfizer observed over 10,000 categories of diagnosis, many of them very severe and very rare. For example:
Pfizer was aware of 73,542 cases of 264 categories of vascular disorders from the shots. Many of them are rare conditions.
There were hundreds of categories of nervous system disorders, totaling 696,508 cases.
There were 61,518 AEs from well over 100 categories of eye disorders, which is unusual for a vaccine injury.
Likewise, there were over 47,000 ear disorders, including almost 16,000 cases of tinnitus, which even Mayo Clinic researchers observed as a common but often devastating side effect early on.
There were roughly 225,000 cases of skin and tissue disorders.
There were roughly 190,000 cases of respiratory disorders.
Disturbingly, there were over 178,000 cases of reproductive or breast disorders, including disorders you wouldn’t expect, such as 506 cases of erectile dysfunction in men.
Very disturbingly, there were over 77,000 psychiatric disorders observed following the shots, lending credence to Dr. Peter McCullough’s research observing case studies showing psychosis correlating with vaccination.
3,711 cases of tumors – benign and malignant
Of course, there were almost 127,000 cardiac disorders, running the gamut of about 270 categories of heart damage, including many rare disorders, in addition to myocarditis.
There were over 100,000 blood and lymphatic disorders, for both of which there’s a wealth of literature linking them to the spike protein.
When reading what Pfizer knew early on juxtaposed to independent studies, it’s clear that nobody could have mistaken most of these AEs for mere incidental ailments. Here is a list of 3,129 case studies chronicling vaccine injury in every organ system observed in this Pfizer document.
What is so jarring is that there are hundreds of very rare neurological disorders that reflect something so systemically wrong with the shots, a reality that was clearly of no concern to the manufacturers and regulators alike. One of the infamous cases of vaccine injury was Maddie de Garay, an Ohio teen who became disabled for life immediately after participating in the Pfizer clinical trial. Her story is chronicled in chapter 16 of my book. I checked this confidential document and found that they knew of 68 cases of her rare diagnosis, chronic inflammatory demyelinating polyneuropathy.
2 notes
·
View notes
Text
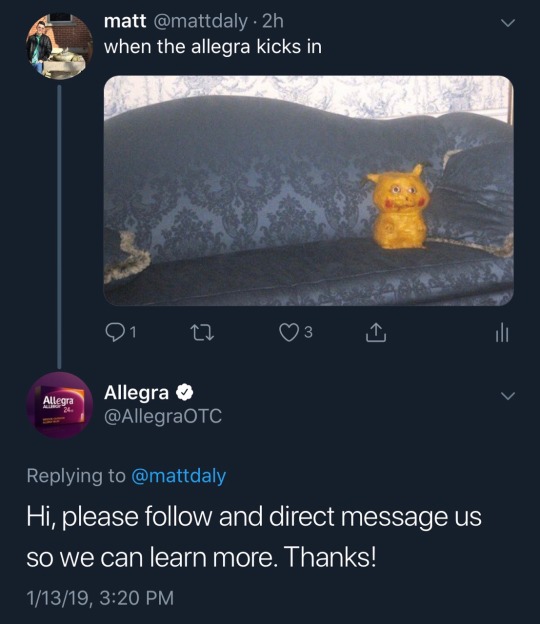
Well actually…. (adjusting my nerd glasses), that‘s not exactly whats happening here. Pharma companies are obligated by law to follow up on adverse events which could be related to the use of their products. This is called pharmacovigilance and very important to help us find out how safe a drug really is. Drugs are tested in clinical trials, which are usually relatively small and only include a subsample of the people who later use the drug in real life. That means we can miss a lot of adverse events, either because they pccur rarely or because we they only happen in a suset of people who have not been included in the clinical trial.
If you work for a pharma company, you have to report any adverse event you encounter. And the companies usually look through social media mention of their products. The follow up is very time consuming but also IT SAVES LiFES! Often the data collected from those types of reports can lead to an adaption to how a drug is prescribed and to label changes.
Which means someone in the PV departement of Allegra had the unglamourous job to follow up on this shit post to find out if it was based on an actual experience.
tldr: report all adverse events to save lifes and annoy pharma companies.
314K notes
·
View notes
Text
Clinical Research Companies in India: Clinfinite Solutions Leading the Way
India has arisen as one of the most noticeable centers for clinical examination, with a quickly developing industry driven by a blend of a tremendous patient pool, an elite foundation, and great administrative structures. Thus, clinical research companies in India are assuming a vital part in the worldwide drug and medical services areas, working with the improvement of new therapies and treatments. One such driving player in this space is Clinfinite Solutions.
Why India is a Hotspot for Clinical Research
India's quickly extending medical services and drug markets, combined with the country's rich variety of hereditary and epidemiological circumstances, make it an optimal area for clinical preliminaries. With an accomplished ability pool, admittance to reasonable assets, and great exploration offices, India gives clinical examination associations (CROs) with one-of-a-kind benefits.
Clinfinite Solutions, situated in Hyderabad, is one of the most believed clinical research companies in India. The organization has a demonstrated history of leading fruitful clinical preliminaries, helping drug and biotechnology organizations carry protected and viable treatments to the market.
Clinfinite Solutions: A Leader in Clinical Research
As a head clinical think-tank in India, Clinfinite Solutions offers a large number of administrations, including clinical preliminary administration, administrative undertakings, clinical information for executives, and pharmacovigilance. With a group of profoundly talented experts and a quality promise, Clinfinite Solutions guarantees that each clinical preliminary fulfills the most noteworthy guidelines of greatness.
Clinfinite Solutions accomplices with both homegrown and worldwide clients, offering start-to-finish support all through the clinical preliminary interaction. Their skill in overseeing huge-scope clinical examinations and their profound comprehension of administrative necessities make them an important accomplice for drug organizations around the world.
Why Choose Clinfinite Solutions?
Aptitude in Clinical Preliminaries: Clinfinite Solutions has long periods of involvement dealing with complex clinical preliminaries, guaranteeing proficient and ideal outcomes.
Worldwide Norms: The organization complies with global administrative rules to guarantee consistency and well-being in clinical examination.
Development and Innovation: With state-of-the-art advancements and imaginative arrangements, Clinfinite Solutions offers unrivaled clinical preliminary administration.
India's clinical examination industry proceeds to flourish, and organizations like Clinfinite Solutions are driving its prosperity. If you're searching for a solid accomplice for clinical preliminaries, Clinfinite Solutions stands apart among the main clinical research companies in India.
0 notes
Text
Unlocking Pharma Insights : Chemxpert Database for Compliance & Market Access
Explore the latest pharma industry trends and streamline your compliance efforts with Chemxpert Database. Access critical resources from the FDA website to stay compliant with GMP in the pharmaceutical industry. Harness the power of data analytics in the pharmaceutical industry to optimize manufacturing and distribution. Simplify pharmaceutical market access strategies and stay ahead in the competitive landscape with Chemxpert’s comprehensive solutions. Your go-to tool for staying compliant and competitive!
#food drug administration#api manufacturers in India#pharmaceutical software companies#pharmacovigilance companies#pharma software companies
1 note
·
View note
Text
#Pharmacovigilance Services#Pharmacovigilance Solutions#Pharmacovigilance Services Providing Company#Pharmacovigilance In USA
0 notes
Text
Contract Research Organization (CROs) Services Market – Industry Trends and Forecast to 2030 Leaders, Graph, Insights, Research Report, Companies

Contract Research Organization (CROs) Services Market Size And Forecast by 2030
According to Data Bridge Market Research Data Bridge Market Research analyses that the Global Contract Research Organization (CROs) Services Market which was USD 50.9 Billion in 2022 is expected to reach USD 109.9 Billion by 2030 and is expected to undergo a CAGR of 10.10% during the forecast period of 2022 to 2030
Our comprehensive Contract Research Organization (CROs) Services Market report is ready with the latest trends, growth opportunities, and strategic analysis. https://www.databridgemarketresearch.com/reports/global-contract-research-organization-cros-services-market
**Segments**
- Based on service type, the Contract Research Organization (CROs) Services market can be segmented into clinical research services, early-phase development services, laboratory services, consulting services, and others. Clinical research services include patient recruitment, site management, data management, pharmacovigilance, and regulatory affairs. Early-phase development services encompass preclinical and Phase I-III clinical trials. Laboratory services involve bioanalytical testing, pharmacokinetics, and biomarker analysis. Consulting services cover regulatory consulting, drug development strategy, and market access support. The ""others"" category may include services such as medical writing, quality assurance, and post-market ing surveillance.
- Geographically, the market can be divided into North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. North America holds the largest market share due to a robust pharmaceutical and biotechnology industry, coupled with a high demand for outsourcing clinical research activities. Europe follows closely behind, with strong regulatory frameworks and increasing investments in research and development activities. The Asia-Pacific region is expected to witness significant growth owing to a rise in clinical trial activities, infrastructure development, and cost-effective services offered by CROs.
- On the basis of therapeutic area, the CROs market can be categorized into oncology, cardiovascular diseases, CNS disorders, infectious diseases, metabolic diseases, and others. Oncology holds a substantial share due to the rising prevalence of cancer and the need for efficient drug development processes. The cardiovascular diseases segment is driven by the increasing burden of heart-related conditions globally. CNS disorders, including Alzheimer's and Parkinson's disease, represent a significant segment due to the growing aging population. Infectious diseases, such as COVID-19, have spurred research activities in this area. Metabolic diseases, like diabetes, also contribute to the market growth.
**Market Players**
- Some of the key players in the Contract Research Organization (CROs) Services market include IQVIA, Laboratory Corporation of America Holdings (LabCorp), PAREXEL International Corporation, Charles River Laboratories, ICON plc, PPD Inc., Syneos Health, Inc., Medpace Holdings, Inc., WuXi AppTec, and PRA Health Sciences. These companies are actively involved in strategic collaborations, mergers and acquisitions, and investments in advanced technologies to expand their service offerings and geographic presence. They focus on enhancing operational efficiency, maintaining quality standards, and fostering long-term relationships with pharmaceutical and biotechnology companies to drive revenue growth in the market.
https://www.databridgemarketresearch.com/reports/global-contract-research-organization-cros-services-market Contract Research Organization (CROs) Services market is witnessing a shift towards a more specialized approach. Market players are increasingly focusing on offering niche services tailored to specific therapeutic areas, such as rare diseases, gene therapies, and personalized medicine. This trend is driven by the need for targeted and efficient research solutions to address the complexities of developing treatments for these specialized areas. CROs are investing in building expertise and capabilities in emerging fields like immuno-oncology, gene editing, and digital health to cater to the evolving needs of pharmaceutical and biotechnology companies.
In addition to traditional services, CROs are also expanding their offerings to include real-world evidence generation, patient-centric clinical trials, and virtual trial solutions. The integration of real-world data and digital technologies is enabling more patient-centric approaches to clinical research, leading to improved recruitment and retention rates, as well as more accurate and timely data collection. This shift towards patient-centricity is expected to drive greater efficiency and effectiveness in clinical trials, ultimately accelerating the drug development process.
Another notable trend in the CROs market is the increasing adoption of decentralized and hybrid trial models. The COVID-19 pandemic has accelerated the adoption of remote monitoring, telemedicine, and direct-to-patient services in clinical trials. These decentralized approaches offer several advantages, including greater patient access, reduced costs, and faster recruitment timelines. As a result, CROs are investing in digital infrastructure and capabilities to support the implementation of decentralized trial strategies and enable seamless collaboration between sponsors, CROs, and patients.
Furthermore, regulatory compliance and data security have become paramount considerations for CROs and their clients. With the increasing volume and complexity of data generated in clinical trials, ensuring data integrity, confidentiality, and compliance with data protection regulations is crucial. CROs are investing in robust data management systems, cybersecurity measures, and compliance protocols to safeguard sensitive information and maintain the trust of stakeholders.
Overall, the Contract Research Organization (CROs) Services market is evolving rapidly to meet the changing demands of the pharmaceutical and biotechnology industry. As the landscape continues to shift towards personalized medicine, digital innovation, and patient-centricity, CROs are poised to play a critical role in driving innovation, efficiency, and quality in clinical research and drug development processes. The market is ripe with opportunities for companies that can adapt to these emerging trends and deliver high-value solutions that address the evolving needs of their clients and the healthcare industry as a whole.**Segments**
Global Contract Research Organization (CROs) Services Market is segmented by Molecule Type, Type, Therapeutic Area, and End User. - **Molecule Type**: The market is categorized into Vaccines, Cell Gene Therapy, and Others. - **Type**: Services include Early Phase Development Services, Pharmacokinetics /Pharmacodynamics, Clinic Research Services, Laboratory Services, Physical Characterization, Stability Testing, Batch Release Testing, Raw Material Testing, Other Analytical Testing, and Consulting Services. - **Therapeutic Area**: Segments include Oncology, CNS Disorders, Infectious Diseases, Cardiovascular Diseases, Immunological Disorders, Respiratory Disorders, Diabetes, and Other Therapeutic Areas. - **End User**: Pharmaceutical and Biopharmaceutical Companies, Medical Device Companies, and Academic Institutes are the primary users of CRO services.
**Market Players**
Notable players in the Contract Research Organization (CROs) Services market include: - PPD Inc. (U.S) - IQVIA Inc (U.S) - ICON Plc (Ireland) - Parexel International Corporation (U.S) - American Preclinical Services, LLC (U.S) - Labcorp Drug Development (U.S) - Theorem Clinical research (U.S) - WuXi AppTec (China) - Syneos Health (U.S) - Evotec SE (Germany) - Charles River Laboratories (U.S) - Medpace (U.S) - FIRMA CLINICAL (U.S) - Frontage Labs (U.S) - Bioagile (India) - Novotech (Australia)
The Contract Research Organization (CROs) Services market is witnessing significant growth and evolution driven by various factors. The shift towards specialized services tailored to specific therapeutic areas like rare diseases and gene therapies is reshaping the market landscape. This trend aligns with the industry's increasing focus on targeted and efficient research solutions to address the complexities of developing treatments for specialized areas, such as personalized medicine.
Moreover, the incorporation of real-world evidence generation, patient-centric clinical trials, and virtual trial solutions is transforming how CROs operate. By leveraging real-world data and digital technologies, CROs can offer more patient-centric approaches to clinical research, leading to enhanced recruitment rates, data accuracy, and overall efficiency in drug development processes.
Another key trend in the CROs market is the adoption of decentralized and hybrid trial models. The COVID-19 pandemic has accelerated the adoption of remote monitoring, telemedicine, and direct-to-patient services, offering benefits such as improved patient access, cost savings, and faster recruitment timelines. As a result, CROs are investing in digital infrastructure to support decentralized trial strategies and enhance collaboration between stakeholders.
Furthermore, ensuring regulatory compliance and data security has become a top priority for CROs and their clients. With the growing volume and complexity of data in clinical trials, robust data management systems, cybersecurity measures, and compliance protocols are crucial to safeguard sensitive information.
In conclusion, the Contract Research Organization (CROs) Services market is dynamic and continuously evolving to meet the changing demands of the pharmaceutical and biotechnology industry. By embracing trends like personalized medicine, digital innovation, and patient-centricity, CROs are playing a crucial role in driving innovation and efficiency in clinical research and drug development processes. Companies that can adapt to these trends and deliver high-value solutions are well-positioned to capitalize on the abundant opportunities present in the market.
The market is highly fragmented, with a mix of global and regional players competing for market share. To Learn More About the Global Trends Impacting the Future of Top 10 Companies in Contract Research Organization (CROs) Services Market : https://www.databridgemarketresearch.com/reports/global-contract-research-organization-cros-services-market/companies
Key Questions Answered by the Global Contract Research Organization (CROs) Services Market Report:
What is the current state of the Contract Research Organization (CROs) Services Market, and how has it evolved?
What are the key drivers behind the growth of the Contract Research Organization (CROs) Services Market?
What challenges and barriers do businesses in the Contract Research Organization (CROs) Services Market face?
How are technological innovations impacting the Contract Research Organization (CROs) Services Market?
What emerging trends and opportunities should businesses be aware of in the Contract Research Organization (CROs) Services Market?
Browse More Reports:
https://www.databridgemarketresearch.com/reports/global-glaucoma-surgical-devices-markethttps://www.databridgemarketresearch.com/reports/global-battlefield-management-systems-markethttps://www.databridgemarketresearch.com/reports/global-danon-disease-treatment-markethttps://www.databridgemarketresearch.com/reports/global-automotive-side-window-sunshades-markethttps://www.databridgemarketresearch.com/reports/global-antiallergics-drugs-market
Data Bridge Market Research:
☎ Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 982
✉ Email: [email protected]
0 notes
Text
Life Science Analytics Market Transformation Trends and Business Growth
The Life Science Analytics Market, valued at USD 9.89 billion in 2023, is projected to reach USD 20.0 billion by 2032, exhibiting a Compound Annual Growth Rate (CAGR) of 7.61% during the forecast period from 2024 to 2032.
Market Overview
Life science analytics encompasses the application of data analysis tools and techniques to the life sciences sector, including pharmaceuticals, biotechnology, and healthcare. These analytics solutions facilitate informed decision-making by transforming vast amounts of raw data into actionable insights, thereby enhancing clinical, operational, and financial outcomes.
Get free sample report @ https://www.snsinsider.com/sample-request/1896
Regional Analysis
The adoption of life science analytics varies across regions:
North America: Leading the market due to advanced healthcare infrastructure, significant R&D investments, and early adoption of innovative technologies.
Europe: Experiencing substantial growth driven by supportive government initiatives and a focus on personalized medicine.
Asia-Pacific: Anticipated to witness the fastest growth, propelled by increasing healthcare expenditures, growing awareness of analytics benefits, and expanding pharmaceutical industries.
Market Segmentation
The Life Science Analytics Market is segmented based on:
Component:
Software
Services
Type:
Reporting
Descriptive
Predictive
Prescriptive
Application:
Research and Development
Clinical Trials
Regulatory Compliance
Sales and Marketing
Supply Chain Optimization
Pharmacovigilance
Delivery Model:
On-demand
On-premises
End-user:
Pharmaceutical and Biotechnology Companies
Medical Device Manufacturers
Research Centers
Third-party Administrators
Key Players in the Life Science Analytics Market and Their Offerings
Allscripts Healthcare LLC – Veradigm
Cerner Corporation – Cerner PowerChart
CitiusTech Inc. – BI-Clinical
Health Catalyst – DOS (Data Operating System)
Inovalon – Inovalon ONE Platform
McKesson Corporation – Clear Value Plus
Saama Technologies Inc. – Life Science Analytics Cloud (LSAC)
Optum Inc. – OptumIQ
SCIOInspire Corp. – SCIO Health Analytics
SAS Institute Inc. – SAS Life Science Analytic
Key Highlights
Advanced analytics tools, including cognitive computing, are revolutionizing data utilization in the pharmaceutical and biopharmaceutical sectors.
The integration of Artificial Intelligence (AI) and Machine Learning (ML) algorithms is enhancing data mining capabilities, leading to improved patient-specific treatment approaches.
Strategic partnerships, such as the collaboration between Moderna and IBM to optimize vaccination programs, underscore the critical role of analytics in healthcare.
Future Outlook
The Life Science Analytics Market is on a trajectory of significant growth, driven by the increasing prevalence of chronic diseases, an aging global population, and the rising demand for personalized medicine. Organizations are leveraging datasets from eHealth, mHealth, and Electronic Health Records (EHRs) to enhance patient care through tailored treatments. The ongoing integration of AI-driven algorithms is expected to further refine data analysis, leading to more precise and effective healthcare solutions. Additionally, strategic alliances and acquisitions are anticipated to expand market reach and capabilities, fostering innovation in analytics for digital transformation.
Conclusion
The Life Science Analytics Market is poised for substantial expansion, underpinned by technological advancements, strategic collaborations, and a heightened focus on data-driven, personalized healthcare solutions. As organizations continue to harness the power of analytics, the potential for improved patient outcomes and operational efficiencies becomes increasingly attainable.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Healthcare Provider Network Management Market
Healthcare Education Market
Healthcare Business Intelligence (BI) Market
Healthcare Chatbots Market
#Life Science Analytics Market Share#Life Science Analytics Market#Life Science Analytics Market Size#Life Science Analytics Market Trends
0 notes
Text
Contract Research Organizations (CROs) in India
Introduction
In the biotechnology and pharmaceutical sectors, India has become a major global center for Contract Research Organizations (CROs). Drug research and clinical trials are increasingly being conducted in India due to its economic benefits, highly qualified workforce, and evolving regulatory environment. The nation's CRO market is growing quickly, assisting pharmaceutical businesses in ensuring regulatory compliance and expediting drug development.
Understanding Contract Research Organizations (CROs)
CROs help with drug development, discovery, and regulatory procedures, offering vital services to pharmaceutical, biotechnology, and medical device industries. These groups assist businesses in conducting research without having to keep costly internal R&D facilities. In contrast to conventional research labs, CROs work under contract and provide specialized knowledge in data administration, regulatory affairs, and clinical trials, which improves the efficiency and economy of drug development.
Growth of the CRO Industry in India
Cost advantages, highly qualified personnel, and regulatory advancements have all contributed to the exponential rise of the Indian CRO sector. When compared to Western nations, India provides excellent clinical research at a significantly lower cost. The government has also enacted laws to facilitate clinical trials and expedite the regulatory procedure. The growth of the CRO industry has been further stimulated by the growing interest of international pharmaceutical corporations in India.
Key Services Offered by CROs in India
Indian CROs offer a wide range of services that span the entire drug development lifecycle. These include:
Preclinical Research: Conducting laboratory and animal studies to evaluate the safety and efficacy of new drugs.
Clinical Trials (Phase I-IV): Managing and executing human trials in compliance with regulatory guidelines.
Regulatory Affairs: Assisting pharmaceutical companies in obtaining approvals from the Drug Controller General of India (DCGI), the U.S. FDA, and other regulatory agencies.
Data Management and Analytics: Using advanced technology and big data to improve research accuracy and decision-making.
Pharmacovigilance: Monitoring drug safety after market launch to ensure public health protection.
Leading CROs in India
Several Indian CROs have gained global recognition for their high-quality research and cost-effective solutions. Some of the leading CROs in India include:
Syngene International: A premier CRO offering integrated drug discovery and development services.
Veeda Clinical Research: Specializes in conducting clinical trials with a focus on regulatory compliance.
Cliantha Research: Provides bioanalytical, clinical research, and dermatology testing services.
Lambda Therapeutic Research: A globally recognized CRO focusing on early-phase clinical trials.
Accutest Research Laboratories: Specializes in bioequivalence and pharmacokinetic studies.
These CROs have contributed significantly to India’s reputation as a leading destination for clinical research.
Challenges Faced by the CRO Industry in India
Despite rapid growth, the Indian CRO industry faces several challenges that need to be addressed for sustainable success:
Ethical Concerns: Ensuring ethical conduct in clinical trials and protecting patient rights remains a priority.
Regulatory Hurdles: Navigating complex approval processes and meeting international standards can be time-consuming.
Competition from Global CROs: Large multinational CROs dominate the market, creating intense competition.
Data Security: Protecting intellectual property and ensuring data privacy is critical for maintaining trust.
Addressing these challenges through better policies, infrastructure, and industry regulations will be key to long-term growth.
The Future of CROs in India
The future of CROs in India is bright due to growing international alliances and technical improvements. Drug research is changing as a result of the combination of big data and artificial intelligence, which increases data accuracy and trial efficiency. Additionally, there is an increasing need for generic and biosimilar medicine testing, which opens up new prospects for CROs. To further solidify its place in the global market, India is now branching out into cutting-edge scientific fields like gene therapy and personalized medicine.
Conclusion
As long as it overcomes obstacles and maintains quality and compliance, India's CRO sector is expected to become even more important in the global drug development process. The nation is a popular location for pharmaceutical research because of its highly qualified workforce, cost benefits, and changing regulatory landscape. Indian CROs are positioned for long-term growth as innovation and technology breakthroughs continue to influence the sector, positioning them as important participants in the future of global healthcare.
India may further establish itself as a top location for contract research by emphasizing ethical behavior, technological integration, and legislative reforms. This will assist to expedite and enhance the delivery of life-saving medications to the market.
#contract research organization in india#clinfinite solutions#best contract research organization in india#indian contract research organization
0 notes